Nplate® is a thrombopoietin receptor agonist indicated for the treatment of thrombocytopenia in adult patients with immune
... Read MoreIn pediatric ITP: For one less thing that may keep your patients from doing what they love
Nplate® is approved for the treatment of thrombocytopenia in pediatric patients 1 year of age and older with ITP for at least 6 months who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
7 out of 10 pediatric patients reached target platelet counts with Nplate®1,*
Phase 3 pediatric pivotal trial (N = 62)1,2
Nplate® was studied vs placebo in a double-blind, 24-week, multicenter, randomized, phase 3 study in pediatric patients 1 year to 17 years with immune thrombocytopenia (ITP).
- Patients were refractory or relapsed after at least one prior ITP therapy with a platelet count ≤ 30 × 109/L.
- Patients were stratified by age and randomized (2:1) to receive a starting dose of 1 mcg/kg subcutaneously weekly either Nplate® or placebo.
- Age categories were ≥ 1 to < 6 years, 6 to < 12 years, and 12 to < 18 years.
- Over a 24-week treatment period, dose was titrated up to a maximum of 10 mcg/kg weekly of either Nplate® or placebo in an effort to maintain a target platelet count of ≥ 50-200 × 109/L.
Pediatric pivotal trial endpoints1,2
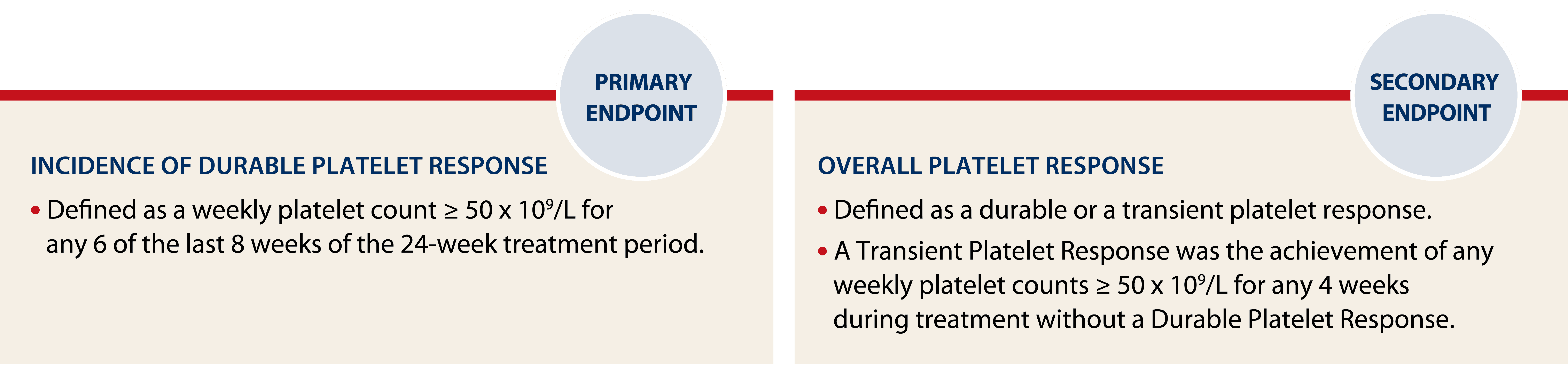
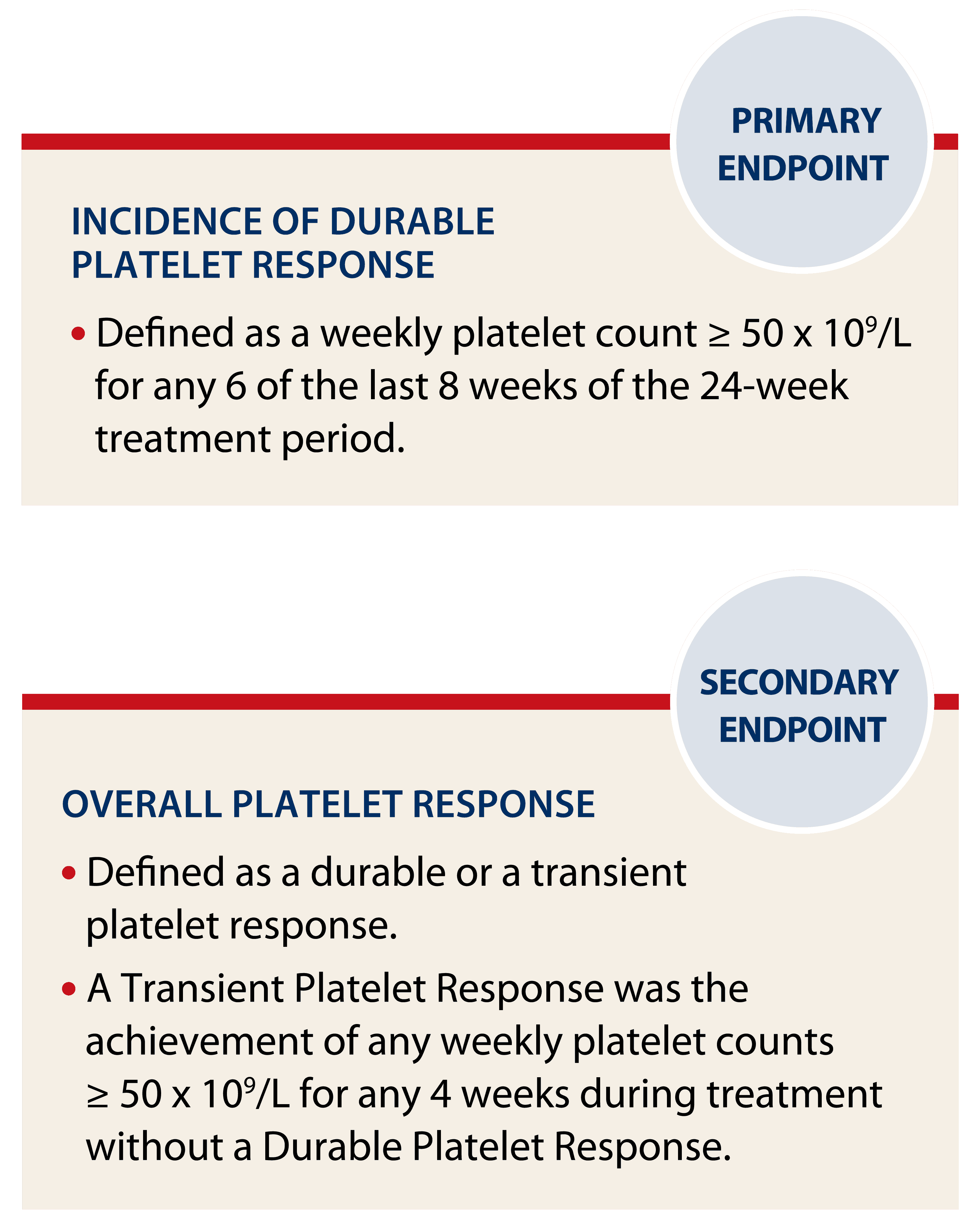
Platelet responses were excluded for 4 weeks after receiving rescue medications.
*Based on Overall Platelet Response.
Platelet response in the 24-week phase 3 pediatric trial1

Achieving and maintaining a platelet count ≥ 50 × 109/L can reduce the risk for bleeding.1
Nplate® was studied in a long-term extension study of up to ~7.5 years in pediatric ITP3,*
Long-term extension trial study design (N = 66)4:
- Pediatric subjects who had previously completed an Nplate® ITP study were eligible to participate in the study.
- Primary endpoints were incidence and exposure-adjusted incidence of adverse events, including clinically significant changes in laboratory values and incidence of antibody formation.
- Secondary endpoint was platelet response to Nplate®.
- Nplate® was administered weekly by subcutaneous injection.
- Consider long-term study design limitations when interpreting results. This study includes patients from multiple prior studies, is not blinded, not controlled, and includes inherent self-selection bias.
*Median study duration was 2.8 years (N = 65).3
*Median study duration was 2.8 years (N = 65).3

Long-term extension study3,4
- ~ 94% of pediatric patients responded to treatment with Nplate®†
- 30-month median duration of response‡
- ~ 7.5 years maximum platelet response‡
†Defined as one or more platelet count ≥ 50 x 109/L in the absence of rescue medication.
‡Response defined as a median monthly platelet count ≥ 50 x 109/L
SAFETY RESULTS: The most frequently occurring (> 45%) adverse events were headache (58.5%), contusion (50.8%), epistaxis, upper respiratory tract infection and vomiting (49.2% each), cough and oropharyngeal pain (46.2% each). The most frequently reported (> 5%) grade 3 or 4 adverse events were thrombocytopenia (9.2%) and headache (6.2%).
Established safety profile across clinical trials1
Pediatric safety profile was established in a 12- and 24-week trial1,*
Adverse reactions with an incidence of ≥ 25% in the two placebo-controlled trials were contusion, upper respiratory tract infection, and oropharyngeal pain.1
Common adverse reactions from two placebo-controlled pediatric studies1,†
(N = 59)
(N = 24)
*The 12-week trial was the phase 1/2 trial. The 24-week trial was the phase 3 trial.
†≥ 5% incidence and ≥ 5% more frequent in the Nplate® arm.
Phase 1/2 study (N = 22)
Nplate® was studied in patients diagnosed with ITP at least 6 months prior to enrollment with a platelet count ≤ 30 × 109/L.1
- Patients were stratified by age and randomized (3:1) to receive (1 mcg/kg subcutaneously weekly) Nplate® or placebo.1
- Over a 12-week treatment period dose was titrated up to a maximum of 10 mcg/kg weekly of either Nplate® or placebo in an effort to maintain a target platelet count of ≥ 50-250 × 109/L.1

No routine monitoring of hepatotoxicity required
Reduce complexity for your pediatric ITP patients
In-office weekly administration allows you to closely monitor your patients’ treatment1
No dietary restrictions
- No calcium or other dietary restrictions
- Does not inhibit iron absorption









Achieve and maintain platelet count goals with weekly dosing and personalized titration1
- Initial dose: 1 mcg/kg based on actual body weight1
- Adjust dose based on platelet response and reassess body weight every 12 weeks1
Refer to the dosing for more information and Prescribing Information for dilution instructions and additional Important Safety Information.
